Go Home
Go To Introduction
This is Book 1
Chapter 1 - Electricity
Chapter 1.2 - The Numbers
Chapter 2 - Sharing and Bonding
Chapter 3 - Voltage
Chapter 3.2 - Voltage Static
Chapter 3.3 - Batteries
Chapter 3.4 - Solar - Others
Chapter 4 - Resistance
Chapter 4.2 - Parallel Resistance
Chapter 4.3 - Voltage Dividers
Chapter 5 - Semiconductor
Chapter 5.2 - PNP NPN Junctions
Chapter 6 - Capacitor
Back To The Guide
To Book 2
Atoms Sharing with HELIUM
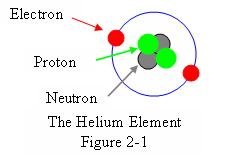
Figure 2.1 illustrates the structure of an atom of Helium, element number 2.
Here the outermost shell, or Valence Shell, in this case the only shell,
has two electrons in orbit. This is the maximum number of electrons that this shell
can hold, so the shell is full.
Helium is one of a set of six special elements known as the Nobel Gases,
each of which has its valence shell filled with electrons.
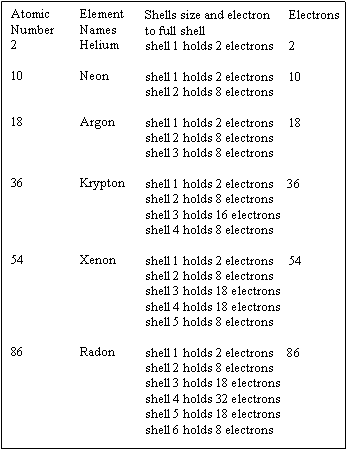
NOBLE GASES
This table is a brief description to these six elements, which are
sometimes called Stable Elements. They are stable because
their valence shell is full, and can not hold any additional electrons.
The six elements Helium, Neon, Argon, Krypton, Xenon, and
Radon are known as the Noble Gases .
The first element, Helium, has one shell and it is full. The next element,
Neon, has two shells and all shells are full, then Argon, Krypton, Xenon,
and finely element Radon with 6 shells with its outer shell full. These
and the rest of the known elements are listed in a Periodic Table .
All the rest of the elements on the periodic table having room in their valence
shell, which means, they can share electrons with other elements.
WOW! What makes this ability to share truly exciting is that this is the
property that gives us the ability to move electrons and get current to flow.
This is where bonding comes in. We all know bonding is a good thing. Every
one of the other 90-plus elements can easily bond with one or more other
elements, by sharing their valence electrons, with other elements.
This is how we get the water we drink, and the air we breathe. Specific to
this book this is how we get our electricity, store electricity and use
electricity. It is this sharing of electrons and the moving and bonding of
electrons that makes it possible for chemical reactions to take place in
batteries, and these reactions make it possible for electricity to flow.
Bonding
When we look at elements that are bonded together we call this arrangement
or mass a Molecule. The structure formed from the bonding of
elements is called Molecular Structure.
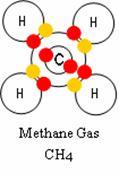
Methane Gas
Such is the case with the combination of elements that makes up the
Methane Gas molecule pictured here.
You can see in this depiction that the Methane molecule is a bonding of
1 Carbon atom, in the center, with its 6 electrons, colored
red. Note that shell one is full, plus there are 4 electrons in orbit in
its outer shell, shell two. This means that shell two has room to share
four additional electrons. It just so happens that Hydrogen
can fill that need, and fill its own outer shell as well. So one carbon
will bond with 4 hydrogen atoms, each with one valance electron, colored yellow.
Take a look at this illustration again. This may help you see why Methane
is one of a number of Hydrocarbon gases?
Covalent Bonding
In the example of Methane Gas, there are 5 atoms that share electron pairs
in an arrangement called Covalent Bonding . That is, each one of
the 4 hydrogen atoms shares its 1 valance electron with 1 valance electron
from the carbon atom. This molecule bonds the 4 hydrogen atoms to the one
carbon atom. Covalent bonding is bonding together of the covalent
electrons in the covalent shell to make the molecule. Recall that the
covalent shell is the outer shell.
These three examples of molecules each has a different bond patterns. In
each of these three examples hydrogen is the single shell element and the
other elements have two shells. You can see in the depiction that all of
the elements shells are full either on their own or by sharing the valence shell space.
The figure on the left has 4 hydrogen atoms bonded with 1 carbon. This should
be familiar to you as our earlier friend, the Methane gas molecule. The center
figure has 3 hydrogen atoms bonded with 1 nitrogen atom and forms Ammonia. The
figure on the right side has 2 hydrogen atoms bonded to 1 oxygen atom, and yes
that is water. You should recall that hydrogen has atomic number 1 on the
periodic table with one electron, and proton. Carbon is number 6 with 6
electron-proton-neutrons sets, nitrogen is number 7 and oxygen is number 8.
Charge it to Atom
Remember that there are several very important subatomic particles to each atom.
In this book I am addressing the electrons over the other subatomic particles.
Moving on, there are situations when a large mass of materials can have extra
electrons. This is sometimes called a charged state. The loss or
gain of electrons does not change the material from one element to another.
But a change in the number of electrons does change the charge of the material.
The atomic number is based on the electron-proton-neutron set of the original
element. For this study, some electrons can come, and some electrons can go,
but each element remains the same.
« Previous Chapter Next Chapter »
Email us: info@shoeboxkits.com