Go Home
Go To Introduction
This is Book 1
Chapter 1 - Electricity
Chapter 1.2 - The Numbers
Chapter 2 - Sharing and Bonding
Chapter 3 - Voltage
Chapter 3.2 - Voltage Static
Chapter 3.3 - Batteries
Chapter 3.4 - Solar - Others
Chapter 4 - Resistance
Chapter 4.2 - Parallel Resistance
Chapter 4.3 - Voltage Dividers
Chapter 5 - Semiconductor
Chapter 5.2 - PNP NPN Junctions
Chapter 6 - Capacitor
Back To The Guide
To Book 2
Energy - On The Move
So what is Electricity? The simple answer is that electricity
is a form of energy not so different from that of sun light or
heat energy. However, with all things in science and nature, a
simple answer does not fully answer the question. Especially
if you really want to study this field. Electricity is the
flow of electrons for one point to another. It is the result
of something else happening. This makes electricity a secondary
source of energy. That is, it takes some primary change in energy
to produce an action, that causes electron movement (electricity).
There are many primary energy changes that cause electrical energy
(electricity). Here are two examples to get you started.
The first example is when a chemical reaction takes place inside
a battery, the primary energy change is the chemical change, and
that change can generate electricity.
The second is when a wire passes through the magnetic field at
either pole of a magnet, with the primary energy being the physical
wire move by the magnetic field and that action caused elecricity
in the wire.
There is more to learn about in each of these actions, but for now,
just reflect on the idea that
one something happens (the primary change)
and that event ends up generating electricity.
The materials in this book will combine some traditional theory,
some chemistry, some mathematics and some easy to build experiments
(kits) to enhance the learners experiences. Take time to look beyond
the surface of the learning and ask yourself how each technology
learning experience can be applied in todays world. Think about
how you might adapt your new-found knowledge in new and different
ways and above all to have fun in the process.
Chemistry and Physics Behind It All (STEM)
Much of science and technology uses fundamental knowledge borrowed
from or shared across many other fields of study. We see this show up
in our every day lives. One example is that we do not consider the
chemistry or the physics that goes into a grocery bag design. We just
want to carry our groceries home from the store. But when that bag rips,
we experience the impact of the decisions someone else made about the
bags materials and its construction.
Electricity is no different. We can flip a switch and the room light
goes on, if we simply want to use the technology without thinking about
it. However, in this study to goal is to understand the inter-workings
of this technology. To do so we need some foundational knowledge to
build upon. As you move through this book, it will provide a cursory
look into other fields in science, technology, engineering, and
mathematics as they relate to the topic at hand.
Starting Off Small:
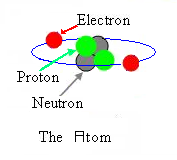
An ATOM is made up of small parts called subatomic
particles. Some of these subatomic particles have names like
electron, proton and neutron. An electron has a negative
(-) change. You can think of each electron as carrying one unit of
negative charge. The electrons counter-part is the proton and it has a
positive (+) charge. A third subatomic particle, the neutron, has no
charge. Except for Hydrogen, which has no neutron, the combination of
two or more protons and that same number of neutrons makes up the
center, also called the nucleus, of the atom.
To keep an atom with a neutral charge it will have the same number of
electrons (negative charge) as it has protons (positive charge). The
electrons are busy flying around the nucleus in orbit called a
cloud or shell.
In earlier scientific studies of atoms and their structure, the scientists
discovered that there is a relationship between the number of shells and
the number of electron-proton-neutron sets. A few basic facts about each
element are listed in a special table of facts called the periodic
table. For example, the first element in the table (atomic number 1)
is Hydrogen with one electron, one proton and one shell. (Hydrogen
has no neutron) The second element in the periodic table is called
Helium. This element (atomic number 2) has a set of two electrons,
two protons, and two neutrons and still only one shell. These two elements,
Hydrogen and Helium are the only two elements with a single shell
arrangement. Atoms with larger atomic numbers may have as many as four, or
five or six electron shells. With the larger numbers of protons and
neutrons in the nucleus, at specific points, an additional shell is needed
to hold the orbiting electrons in their respective orbits. Regardless of
the number of shells, only the outermost shell in any element is called the
valence shell, and the electrons in that shell are called the
valance electrons.
Before we dig into the topic any further, we will want to have a basic
understanding of how numbers are used in electronics.
« Previous Chapter Next Chapter »
Email us: info@shoeboxkits.com